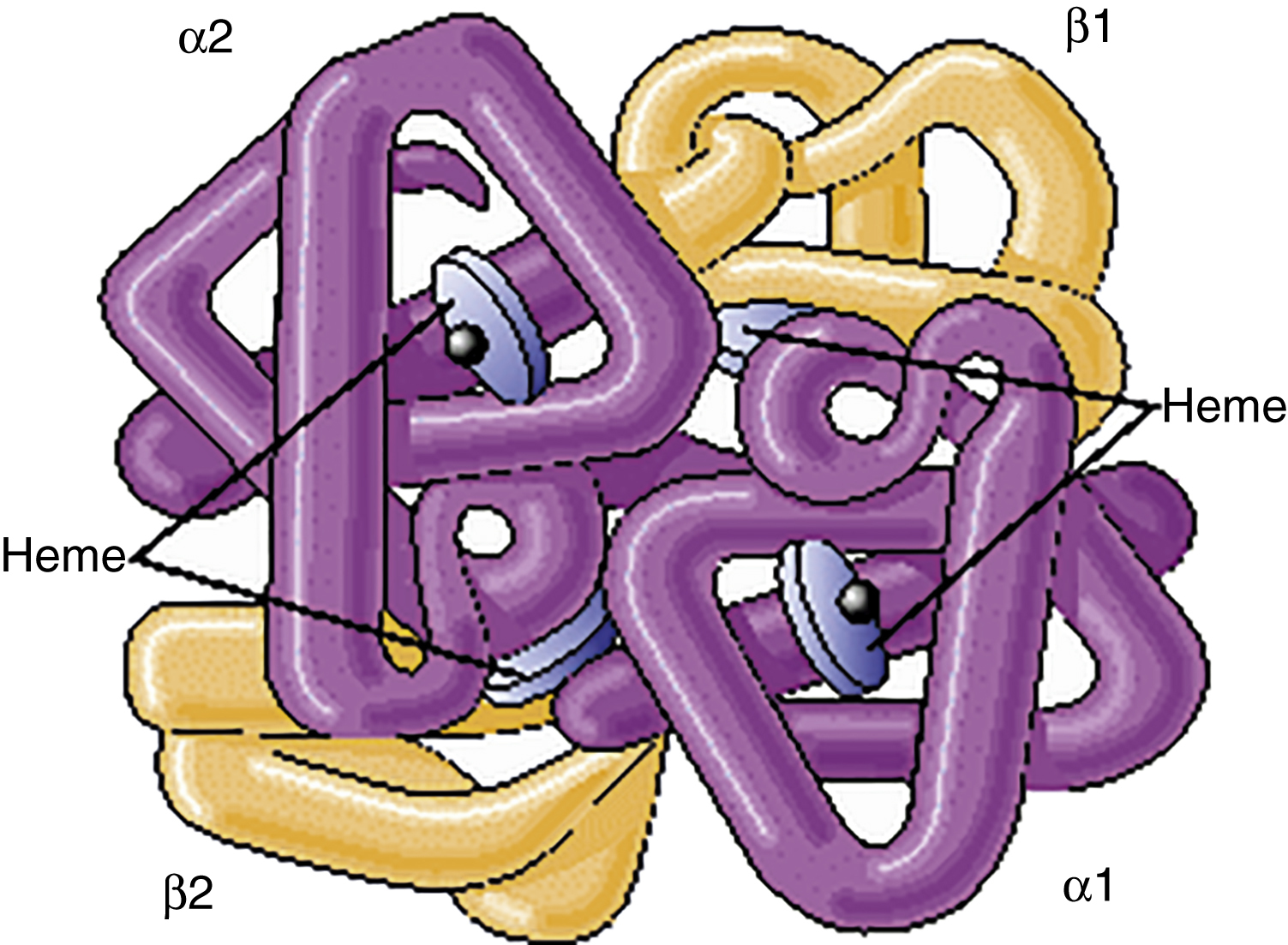
hemoglobin (Hb, Hgb) /hē″məglō′bən/ [Gk, haima + L, globus, ball] , a complex protein-iron compound in the blood that carries oxygen to the cells from the lungs and carbon dioxide away from the cells to the lungs. Each erythrocyte contains 200 to 300 molecules of hemoglobin, each molecule of hemoglobin contains four heme groups, and each heme carries one molecule of oxygen. A hemoglobin molecule contains four globin polypeptide chains. Each polypeptide chain is composed of 141 to 146 amino acids. The absence, replacement, or addition of only one amino acid modifies the properties of the hemoglobin. Different kinds of hemoglobin are identified by their specific combination of polypeptide chains. Normal adult hemoglobin is composed of alpha and beta chains. About 2% of adult hemoglobin is composed of alpha and delta chains, called hemoglobin A2 (Hb A2). Fetal hemoglobin is composed of alpha and gamma globin. The normal concentrations of hemoglobin in the blood are 12 to 16 g/dL in women and 13.5 to 18 g/dL in men. In an atmosphere of high oxygen concentration, such as in the lungs, hemoglobin binds with oxygen to form oxyhemoglobin. In an atmosphere of low oxygen concentration, such as in the peripheral tissues of the body, oxygen is replaced by carbon dioxide to form carboxyhemoglobin. Hemoglobin releases carbon dioxide in the lungs and picks up oxygen for transport to the cells. Also spelled haemoglobin. See also carboxyhemoglobin, complete blood count, erythrocyte, erythropoiesis, heme, hemoglobinopathy, hemolysin, oxyhemoglobin.